

Medical Homepage
Contrarily to fossil-based plastics, our bio-based products bind atmospheric carbon. With our materials, the environmental footprint of your products may be reduced as much as 60-80%. We have the Life Cycle Assessment (LCA) know-how that is needed for application-specific calculations.

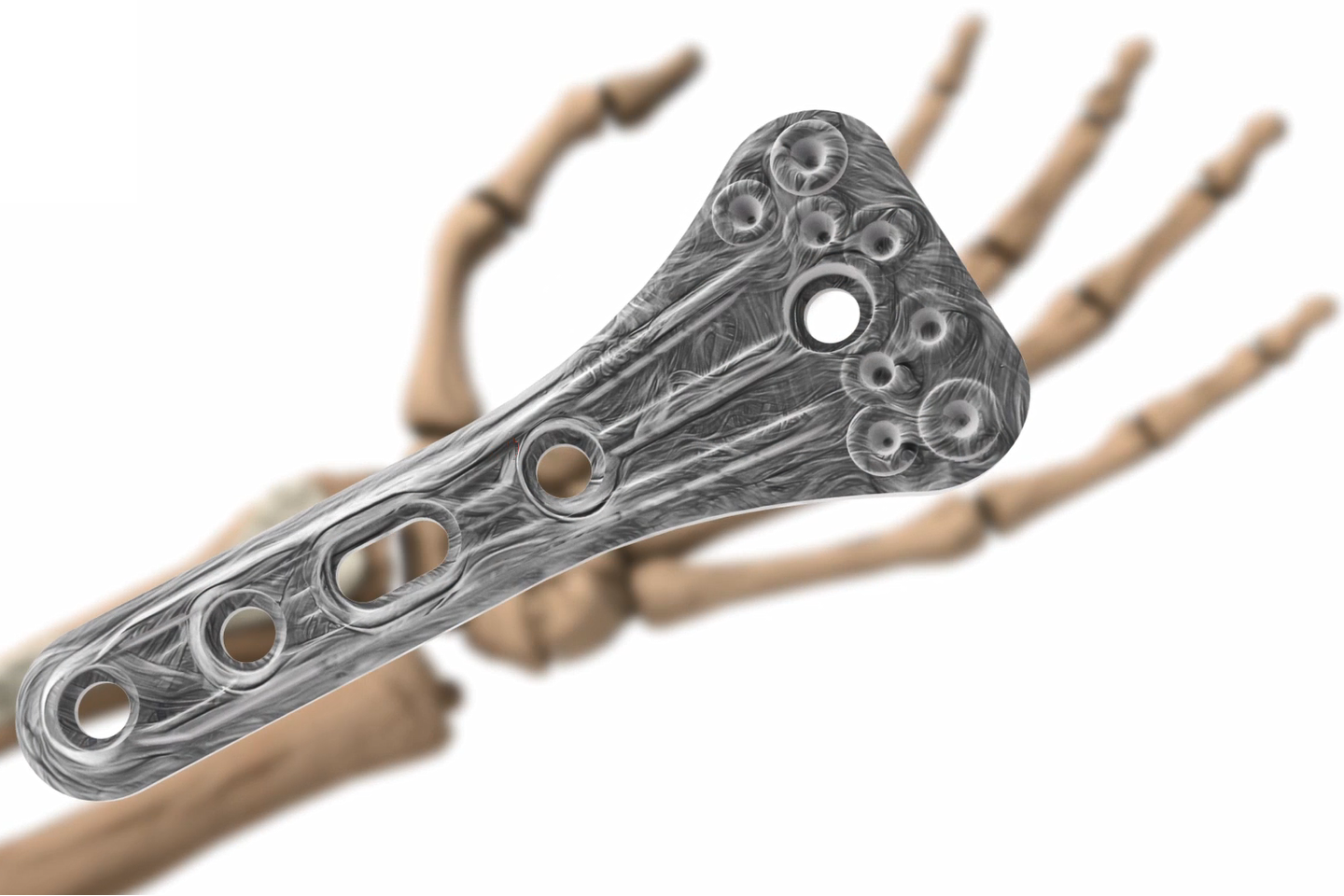
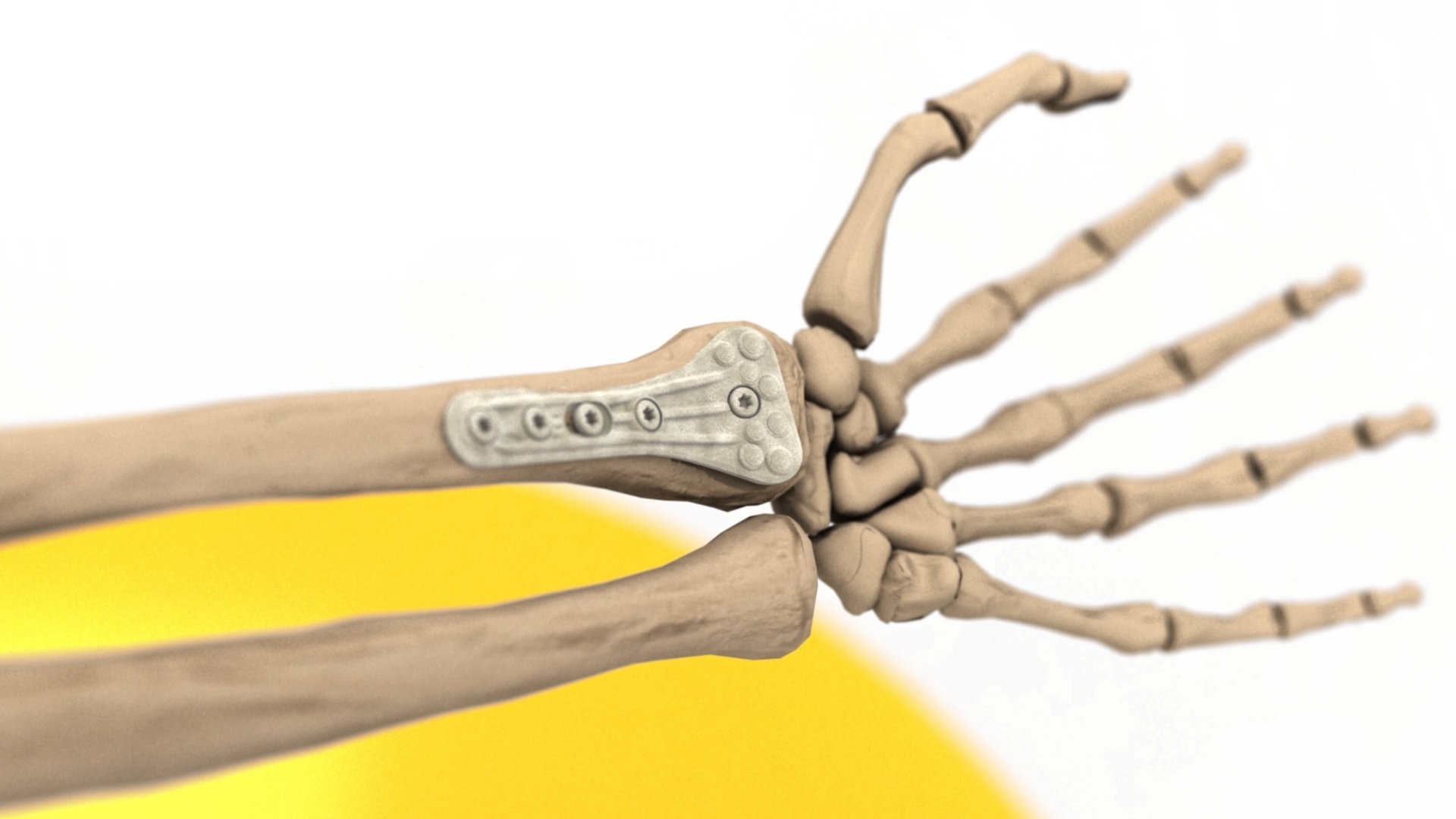
Arctic Biomaterials produces high-quality, strong biomaterials for orthopedic implants and various other healthcare indications (Medical). ABM´s unique natural mineral fiber (or fibre) provides an extremely strong and osteoconductive ingredient to combine with bioresorbable polymers and other biomaterials. The combination offers a composite raw material with additional benefits, meant even for load-bearing applications.
ABM´s natural mineral fiber composite has excellent properties. Unique ultra-high torque strength and advanced bioactivity, bone growth and new bone formation. Learn more HERE
Arctic Biomaterials also produces sustainable alternatives for fossil oil-based plastics with our strong biomaterials (Technical). The technical unit produces high-performance bio-based biodegradable composites for consumer and industrial applications. The added value is related to the increased temperature resistance and mechanical strength. Learn more HERE
Join our journey where material innovations provide unseen product opportunities and a healthy planet!
has a strong knowledge of strong biomaterials, processing, and product design for each application. We have assembled a talented and experienced team. All key persons have more than 15 years of experience in developing and manufacturing biomaterials for orthopedics and other medical devices.
We use our world-class science and engineering expertise to create bioresorbable load-bearing materials. The aim is to replace metals and help our customers to take advantage of demanding market opportunities.
Our goal and driving ambition is to be the trusted partner of choice. We deliver high-value biodegradable composite materials and solutions to our clients. In addition, use our world-class science and engineering to seize market opportunities. Together we solve some of the world’s biggest challenges in resorbable polymer chemistry and processing of strong biomaterials.
Since bioplastics entered technical application and medical device markets, they have lacked the mechanical and/or thermal properties. Arctic Biomaterials came up with innovative solutions through its ABMcomposite technology. We close the property gap between bioplastics and non-biodegradable plastics.
This is the first time that strong bioresorbable materials will be used in load-bearing applications. Manufacturers will be given the design freedom to make competitive implants for titanium or metal alloys. ABMcomposite technology will open also totally new indications and markets for bioresorbable materials.
ABMcomposite technology will for the first time introduce strong biomaterials. They can truly compete with traditional composites made using conventional processing techniques. This is due to the materials having comparable processability, high heat resistance, and excellent mechanical properties.

