Arctic Biomaterials has been developing for its customers an ultra-high strength resorbable materials based on continuous natural mineral fibers, the X3 Fiber. In addition, the patented automated fiber placement (AFP) manufacturing. The bioactive, bio-integrative and bioresorbable X3 fiber composite material is based on ABM´s own natural mineral fiber made with thermoplastic long fiber pultrusion technology. ABM´s unique bioactive natural mineral fiber (fibre), X3 provides an extremely strong and osteoconductive ingredient to combine with medical device manufacturers bioresorbable materials. The absorption time can be tailored by composition selection and processing.
The fiber composite forms a raw material with higher level benefits. This innovation enables unforeseen, ultimate high mechanical properties for resorbable devices (see graphs below), even for load-bearing applications. As the composite is osteoconductive and osteostimulative, it allows an excellent bone-implant interaction and stimulation of osteogenesis. The X3 fiber gives the same benefits as the ceramics but with higher strength. The fibers stimulate osteoblasts to support the producing of a new bone in a bony environment. A balanced pH environment, promoting bone healing and improving biocompatibility.
All the medical grade Evolvecomp™ materials show excellent biocompatibility according to ISO 10993-1 standard. Read more about biocompatibility
Commercialize your vision of strong bioresorbable and bioactive implants using the X3 technology with and ABM´s technical support!

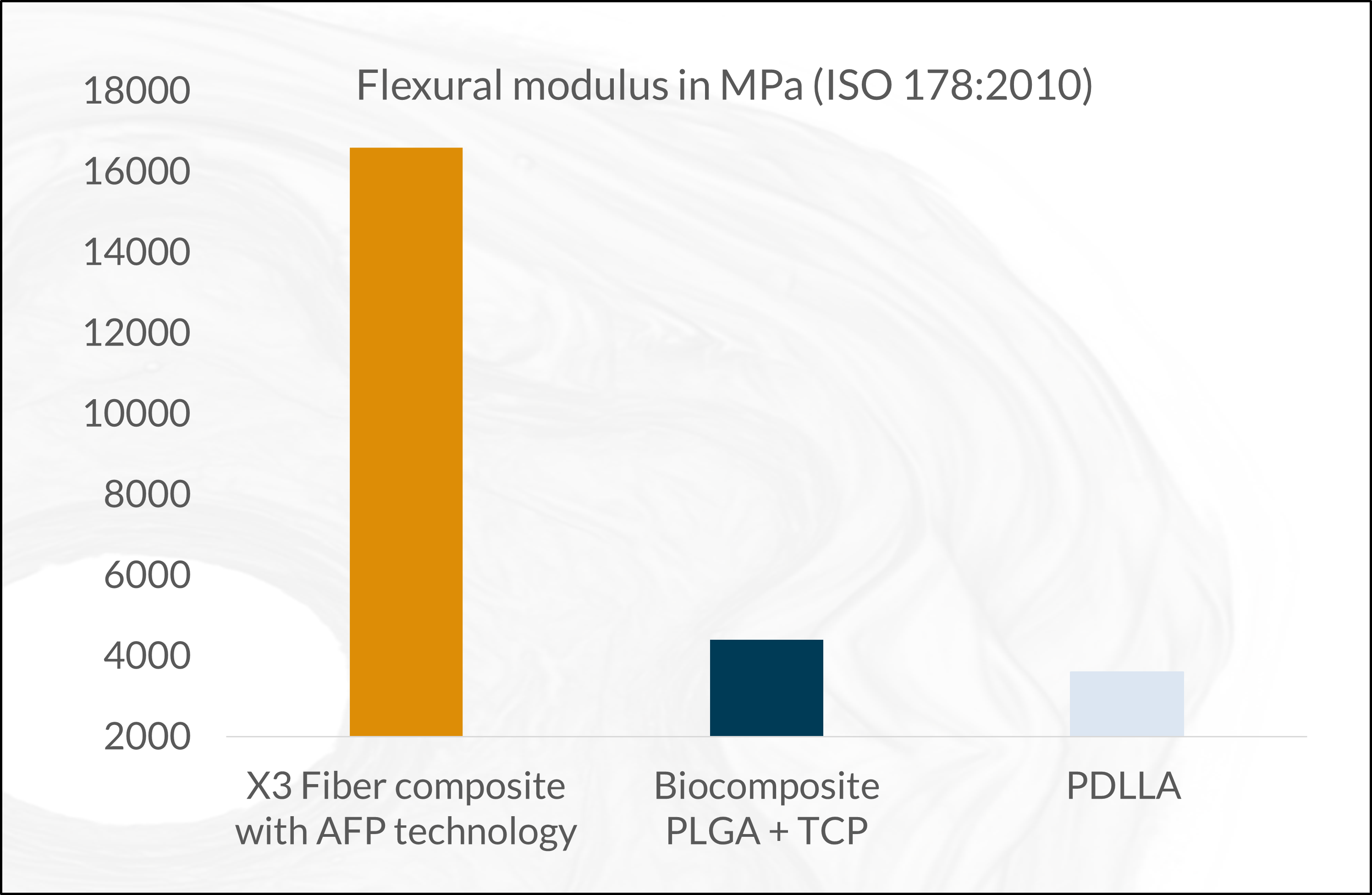
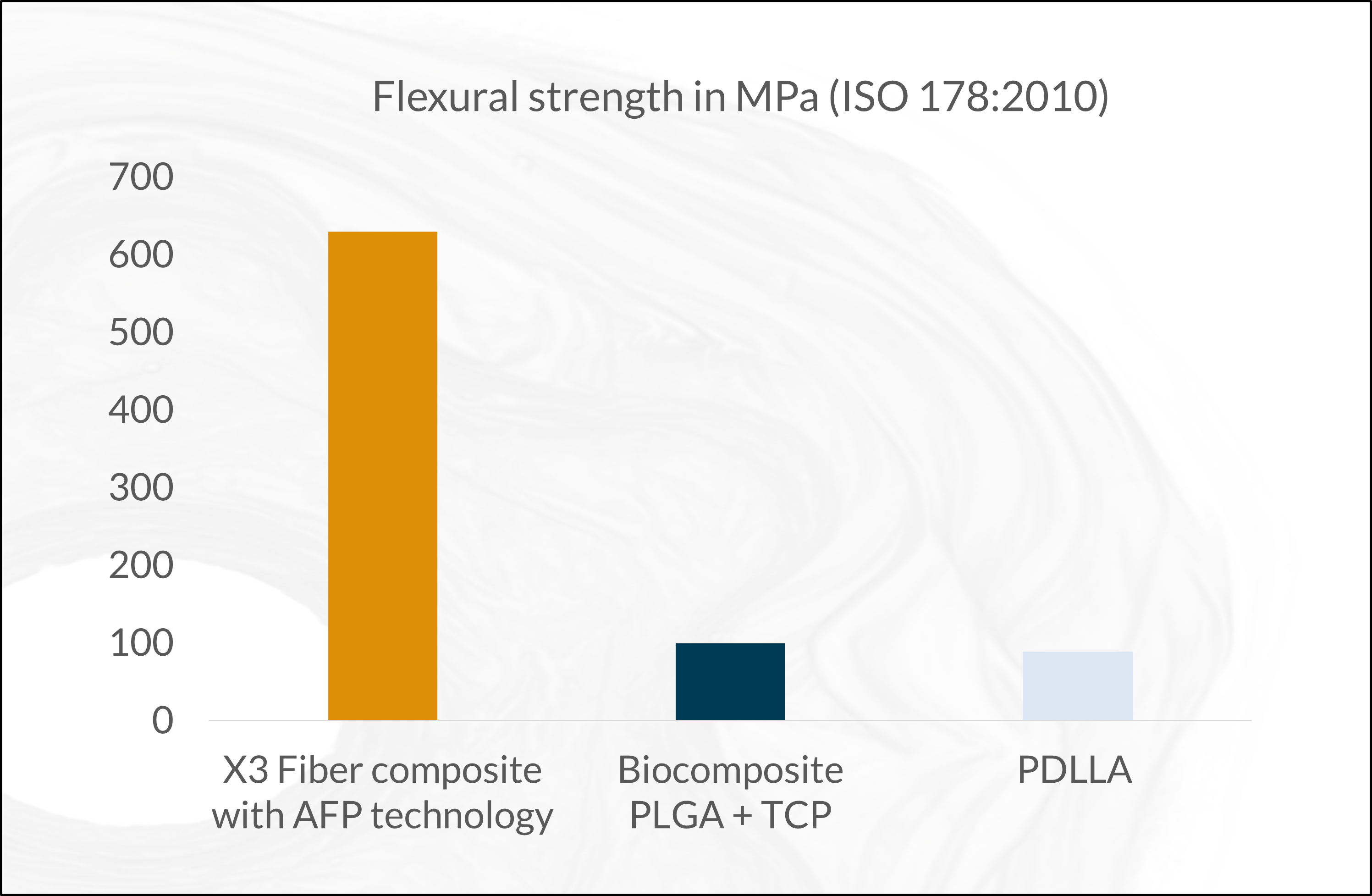
Data and figures from Arctic Biomaterials Research and Development
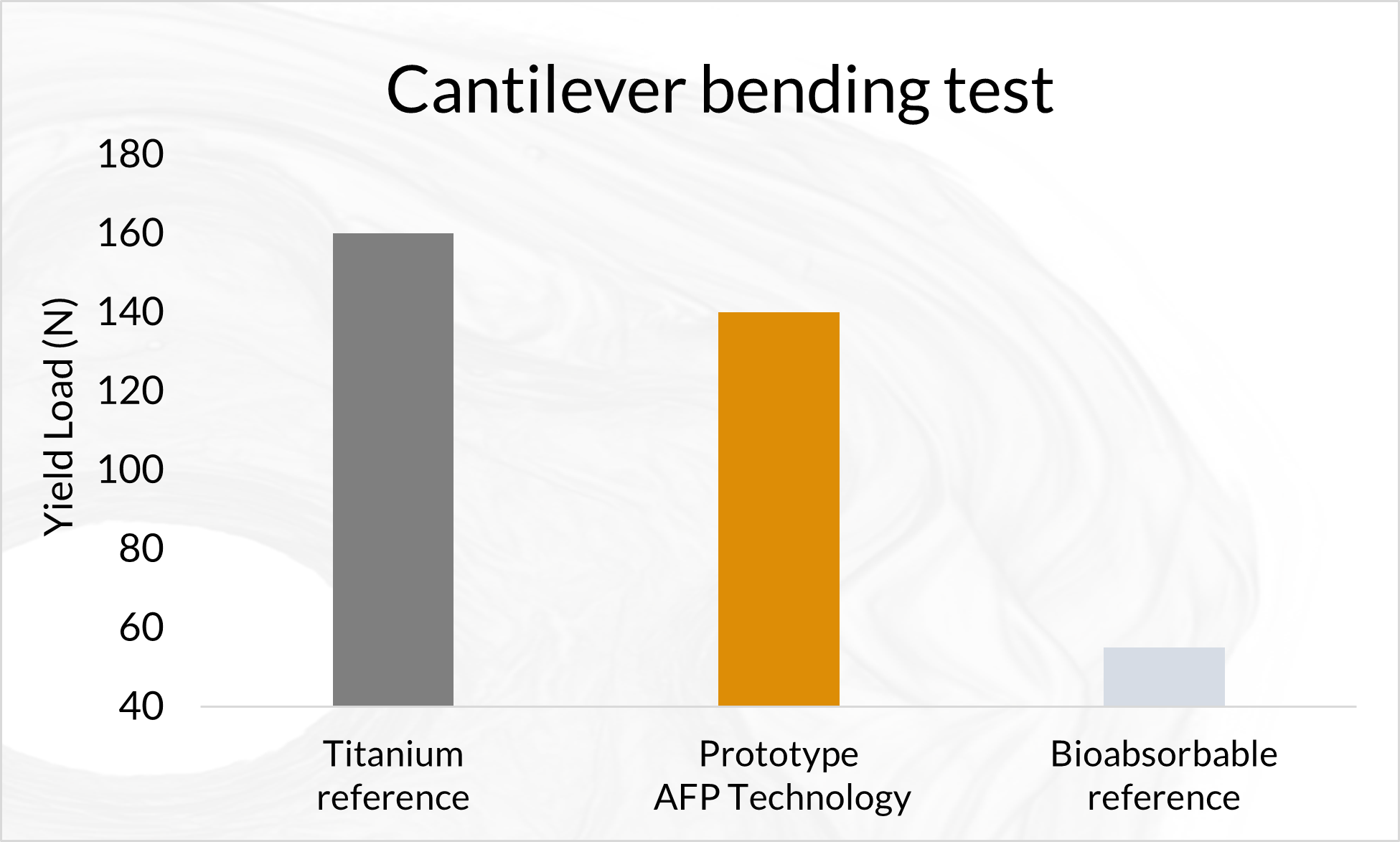
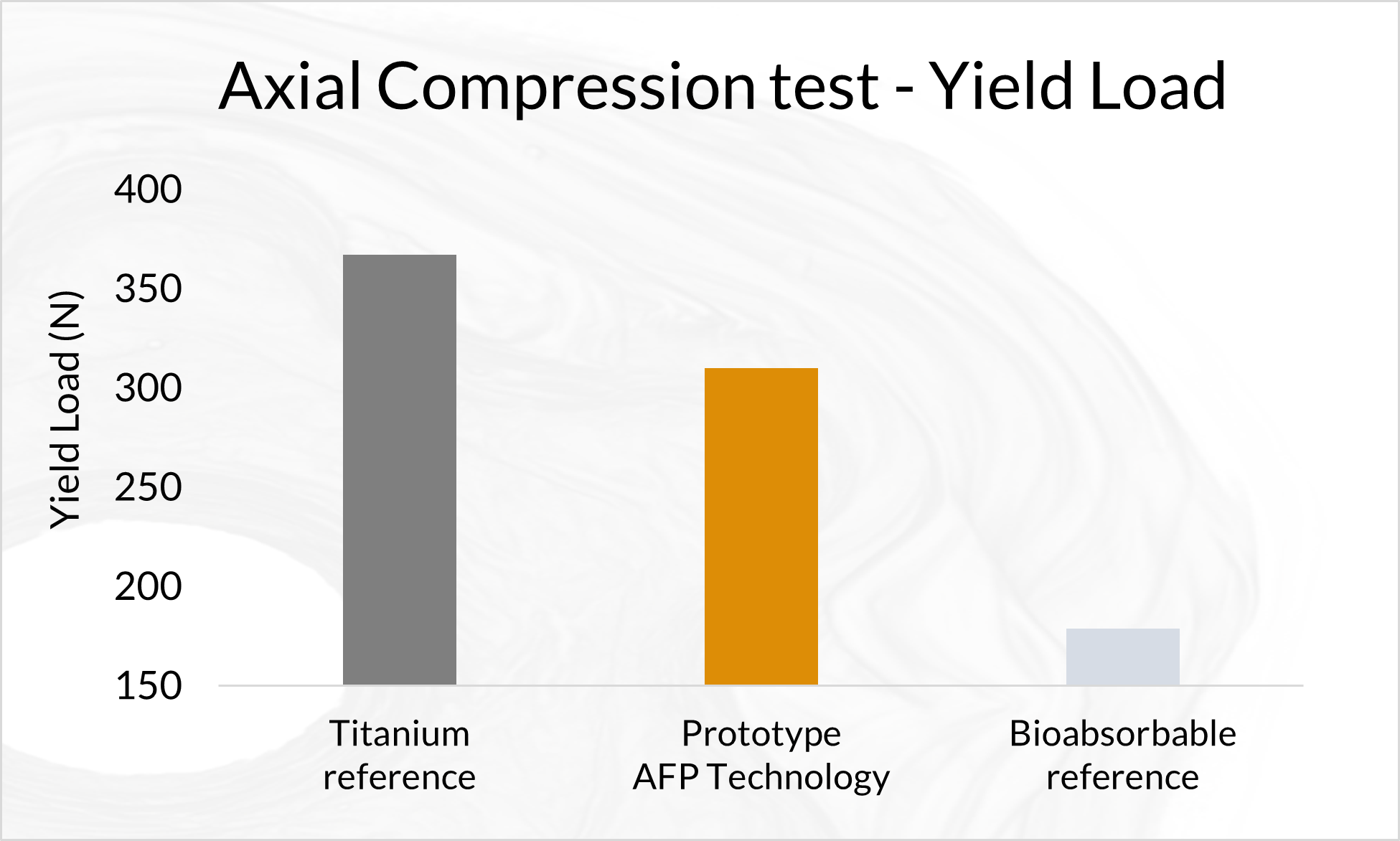
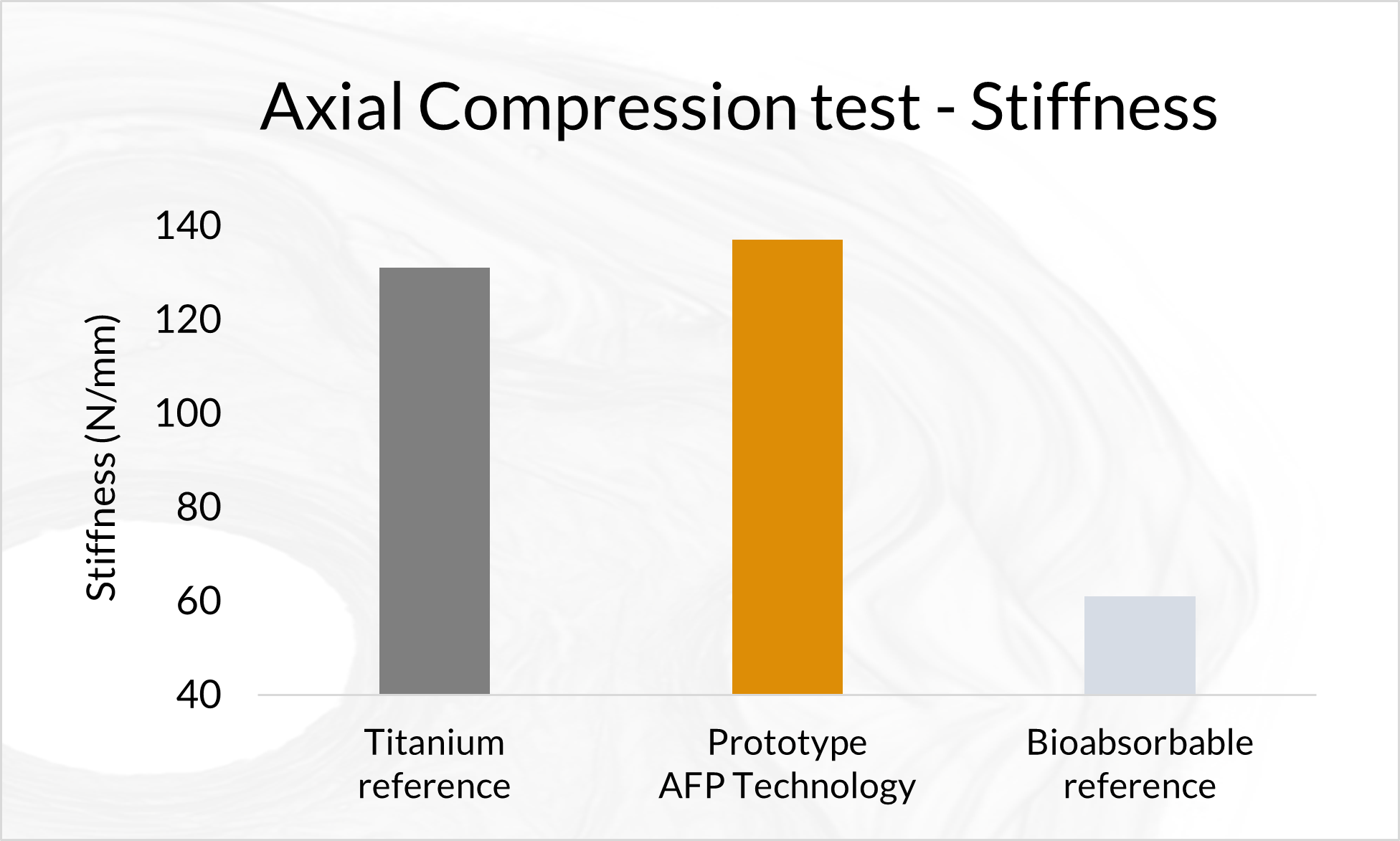
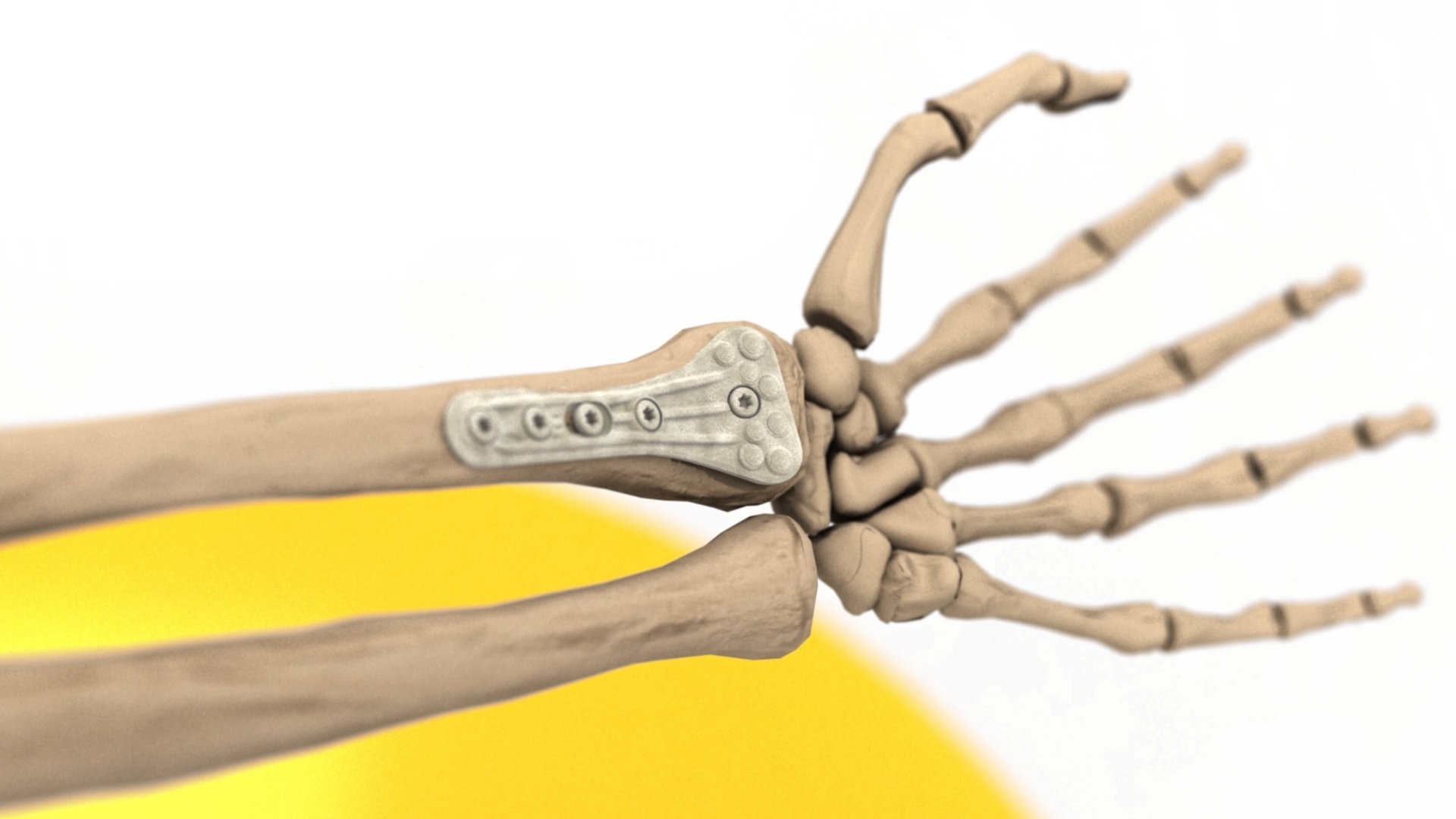
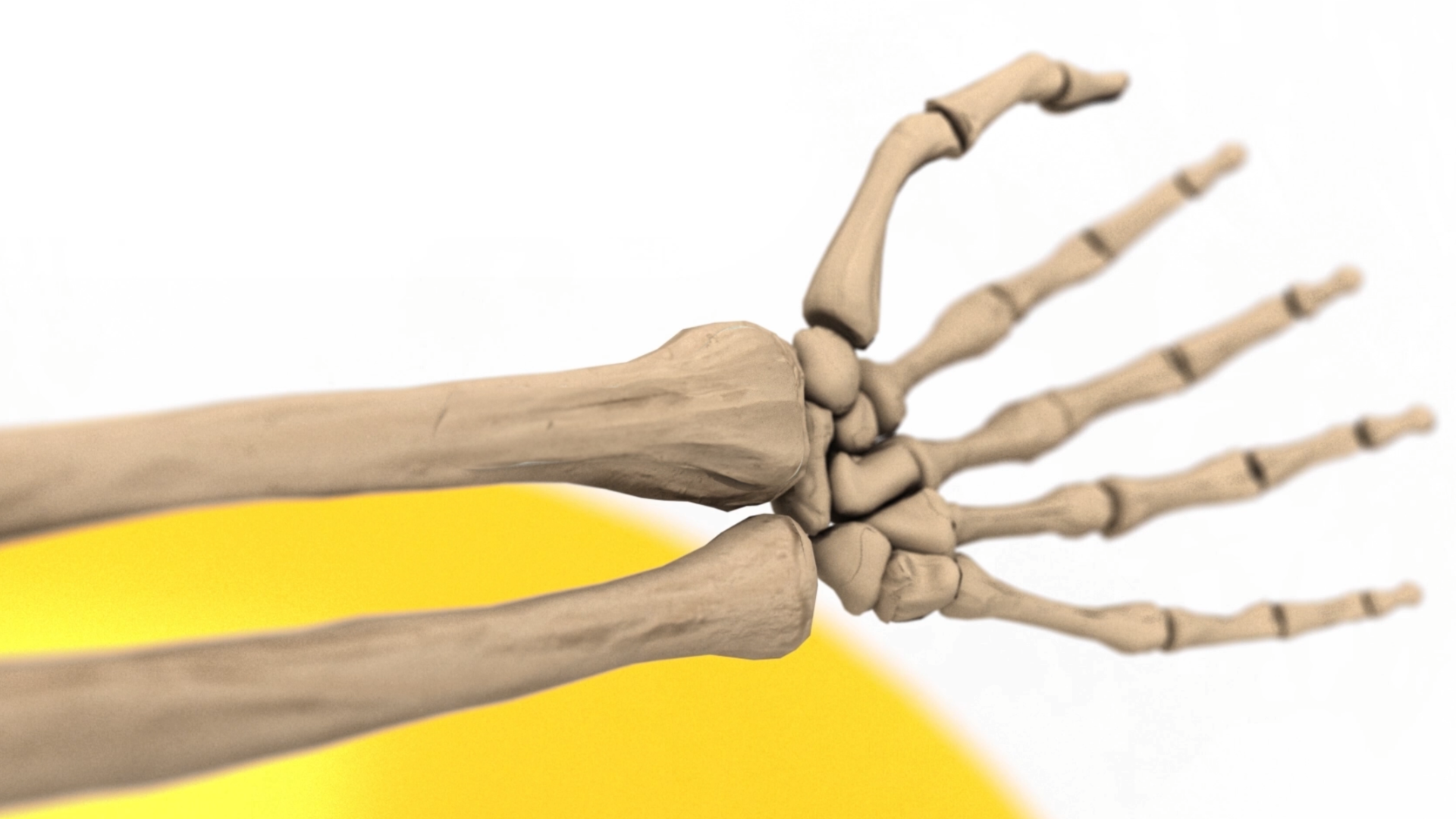
Biomechanical Testing of Prototype AFP Distal Radius Plate – Cantilever Bending and Axial Compression
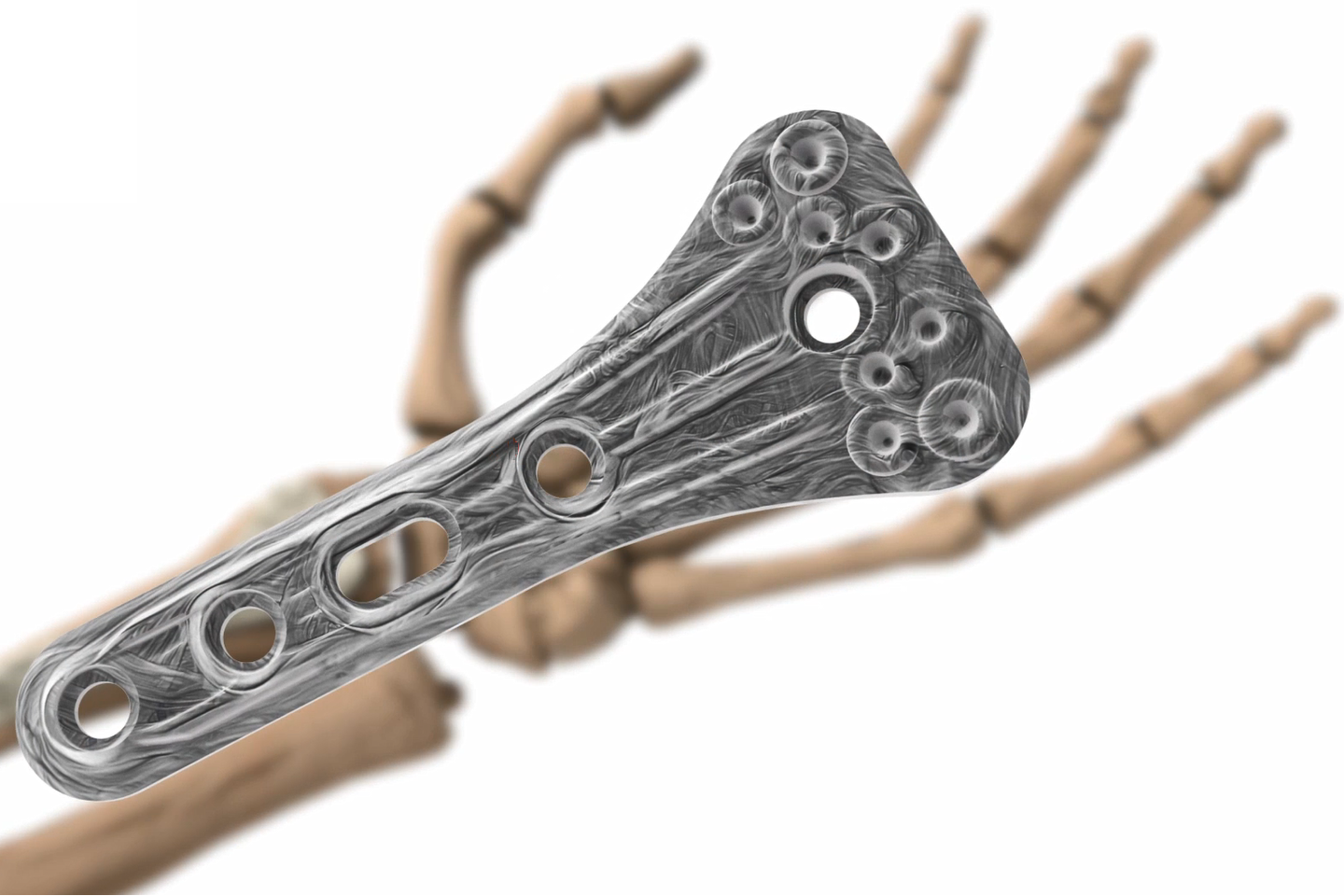
AFP Distal Radius Plate is a prototype bioabsorbable fixation plate manufactured using Arctic Biomaterial’s proprietary Automated Fiber Placement (AFP) technology. The objective of the study was to compare the mechanical properties of AFP Radius Plate with a commercial bioabsorbable radius plate and commercial titanium radius plate of similar sizes.
In the cantilever bending test comparison with commercial titanium plate reference, the mechanical properties are over 85% of the titanium reference strength. The biomechanical axial compression gives the AFP plate the strength that is in average 84% of the titanium reference. In conclusion, the Prototype Distal Radius Plate appears to have initial mechanical properties suitable for load-bearing indications. All the results presented in this paper pertain to an example case using a prototype implant design. When proving the behavior of actual products composed by AFP Technology, similar studies, and possible further analyses need to be conducted in order to verify the mechanical and degradation characteristics of actual products.
More data at White Papers and Literature