White Papers
Stiffness comparison between commercial titanium metacarpal plate and bioresorbable X3 fiber composite prototype plate
Arctic Biomaterials Research and Development
X3 fiber composite prototype plate is a prototype bioresorbable fixation plate manufactured using Arctic Biomaterial’s X3 fiber composite technology. The objective of the study was to investigate the initial stiffness properties of X3 fiber composite prototype plate compared to a commercial metacarpal plate. The initial stiffness of the bioresorbable X3 fiber composite plate with this prototype design was 11% higher than the commercial titanium metacarpal plate. In conclusion, the bioresorbable X3 fiber composite prototype plate appears to have a similar initial stiffness compared to similar-sized commercial titanium metacarpal plate.

X3 fiber composite prototype plate and titanium metacarpal plate, side by side.
Bioactivity of continuous natural mineral fiber reinforced composite pins and plain X3 natural mineral fibers
Arctic Biomaterials Research and Development
These studies demonstrated bone-like apatite layer deposition on the natural mineral fiber reinforced pins and plain X3 natural mineral fibers and the deposit was similar to bone mineral. The results indicate that the X3 bioactive natural mineral fibers in the composite enhance bioactivity.
Composites
In the SEM/EDX analysis, calcium phosphate (CaP) deposits were observed on the surfaces of all samples. When the deposits on fiber reinforced composites and plain copolymer samples were compared, it was found that only the deposit on the fiber reinforced composite pins showed the properties of bone-like apatite and thus bioactivity. Formation of bone-like apatite layer is seen as proof of bioactivity and the ability to bond to bone of bioactivity and the ability to bond to bone. According to Kokubo and Takadama (reference in the White paper) a material that is able to form apatite (a form of calcium phosphate, CaP) on its surface when immersed in SBF (simulated body fluid) is also able to form apatite in a living body and bonds to living bone through this apatite layer as long as the material does not contain any toxic or antibody inducing agents. Thus, the apatite forming ability can be considered as proof for bioactivity.
X3 natural mineral fibers
The results of the plain natural mineral fiber dissolution tests showed that CaP layer was formed around the natural mineral fibers. The deposits and EXD analysis of the CaP layer are shown in figures. When the Ca/P ratio of the deposited clusters was studied, it was noticed that it decreased with time from 3.2 at 26 weeks to 1.61 at 104 weeks. This is slightly lower than the stoichiometric Ca/P ratio of apatite and thus corresponds to the requirements of ISO 23317:2014. Additionally, sodium, chlorine, magnesium, carbon and oxygen were found, which also match with the properties of biological apatite. The crystallinity of the deposits was not studied in this study. However, these results suggest that the CaP layer is matured to apatite with time.
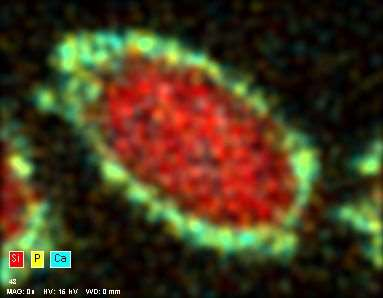
EDX analysis of the 52 week time point natural mineral fiber cross section showing the CaP layer on the surface (Ca in blue and P in yellow).
Biomechanical testing of prototype AFP Distal Radius Plate – Cantilever Bending and Axial Compression
Arctic Biomaterials Research and Development
AFP Distal Radius Plate is a prototype bioabsorbable fixation plate manufactured using Arctic Biomaterial’s proprietary Automated Fiber Placement (AFP) technology. The objective of the study was to compare the mechanical properties of the AFP Radius Plate with a commercial bioabsorbable radius plate and a commercial titanium radius plate of similar sizes.
The mechanical properties tested for the Prototype Distal Radius Plate manufactured using AFP technology are close to the titanium plate reference. In the cantilever bending test comparison with commercial titanium plate reference, the mechanical properties are over 85% of the titanium reference strength. The biomechanical axial compression gives the AFP plate the strength that is on average 84% of the titanium reference.
In conclusion, the Prototype Distal Radius Plate appears to have initial mechanical properties suitable for load-bearing indications. All the results presented in this paper pertain to an example case using a prototype implant design. When proving the behavior of actual products composed by AFP Technology, similar studies, and possible further analyses need to be conducted in order to verify the mechanical and degradation characteristics of actual products.
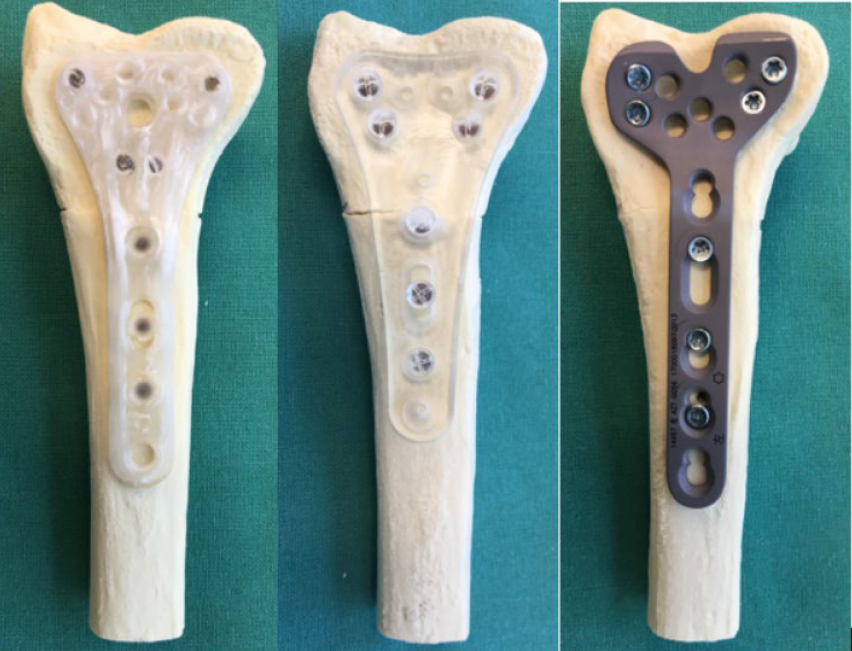
Biomechanical testing samples for distal radius AFP with polymer implant, titanium implant and X3 fiber composite implant
Long-term in vivo properties of natural mineral fiber reinforced composite implants
Arctic Biomaterials Research and Development
Many beneficial properties of silica-based natural mineral fibers are well known and well reported in the literature. Composite materials combine the good properties of bioactive natural mineral fibers and bioabsorbable polymers offering greatly improved mechanical properties compared to implants made of plain polymers. This white paper summarizes a two-year rabbit study ofinjection molded composite samples.
As a conclusion, the biocompatibility and bone-growth promoting properties of the natural mineral fiber reinforced PLDLA composites were studied in a two-year rabbit study. The findings show that the composite implants do not elicit any adverse reactions in the surrounding tissues thus showing good biocompatibility. Additionally, bone ingrowth into the implants was seen although the implanted materials were not completely degraded within the time frame of this study. This study showed that natural mineral fiber reinforced composite materials are very promising bioabsorbable materials for clinical use in bonerelated applications. The results indicate good biocompatibility.
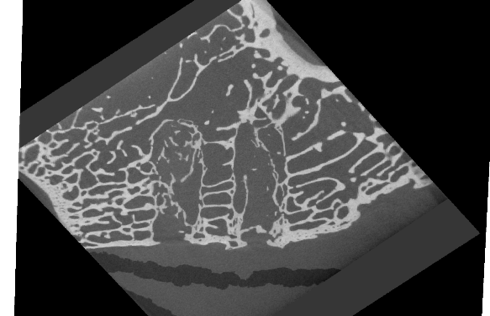
Bone tissue confirmed in a microCT
Degradation characteristics of injection molded Evolvecomp GF40PLD96 products
Arctic Biomaterials Research and Development
This white paper presents degradation properties of investigational interference screw prototypes composed of a blend of Evolvecomp GF40PLD96 pellets and 70L/30D,L PLA (RESOMER LR 708) granules. The outer dimension of the implant prototypes gradually increased during the in vitro degradation, ending up 14 – 18% higher than the initial diameter at week 52. The mass loss started between weeks 26 and 52, being 10 – 25 % of the initial mass at 104 weeks. The largest implant prototypes retained their mass slightly longer than the smaller implant prototypes. Reduction of inherent viscosity during degradation followed similar trend regardless of the implant size and slight variations in initial chemical properties. Shear strength retention analysis revealed that for the first 16 weeks of in vitro degradation, the mechanical properties remained above 80% of the initial wet strength.
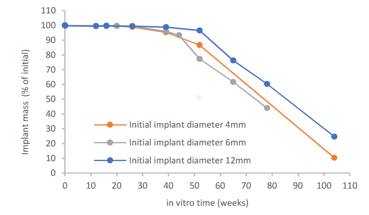
Evolvecomp implant mass loss in vitro
Hydrolytic degradation of the continuous fiber composites of the EvolvecompTM family
Arctic Biomaterials Research and Development
Conventional bioabsorbable polymer implants have lacked the mechanical properties to be applied in load bearing applications in human body. Bioactive natural mineral fiber reinforced composites offer improved mechanical properties. Additionally, bioactive natural mineral fiber component offers another great advantage, bioactivity. This means that it promotes bone healing and bone growth. Continuous fiber reinforced composites elevate the bioabsorbable implant material properties to a whole new level. This white paper summarizes hydrolytic degradation properties of the three composites belonging to the Evolvecomp™ composite family with the Arctic Biomaterials’ proprietary bioactive X3 natural mineral fiber as continuous fiber reinforcement.
Overall, the GF40PLG85 composite degraded faster than the GF40PLD96 and GF40PLD85 composites. This was an expected outcome because PLGA is known to degrade faster than PLDLA. The Evolvecomp™ GF40PLG85 is suitable for applications where fast degradation is desired whereas the other two composites are more suitable for use in applications where good mechanical property retention for longer time periods is wanted.

SEM image of Evolvecomp GF40PLD96 composite
Effect of EtO and NO2 sterilization on injection molded EvolvecompTM products
Arctic Biomaterials Research and Development
The objective of this study was to analyze the effect of two different sterilization methods, NO2 and ethylene oxide (EtO) sterilization, on investigational interference screw type implants. The analyzed products were composed of a blend of Evolvecomp™ GF40PLD96 and 70L/30D,L PLA (Resomer LR708). As a conclusion, EtO sterilization and NO2 sterilization had no remarkable effect on analyzed initial properties or on in vitro degradation characteristics of investigational chopped fiber interference screws type implants.
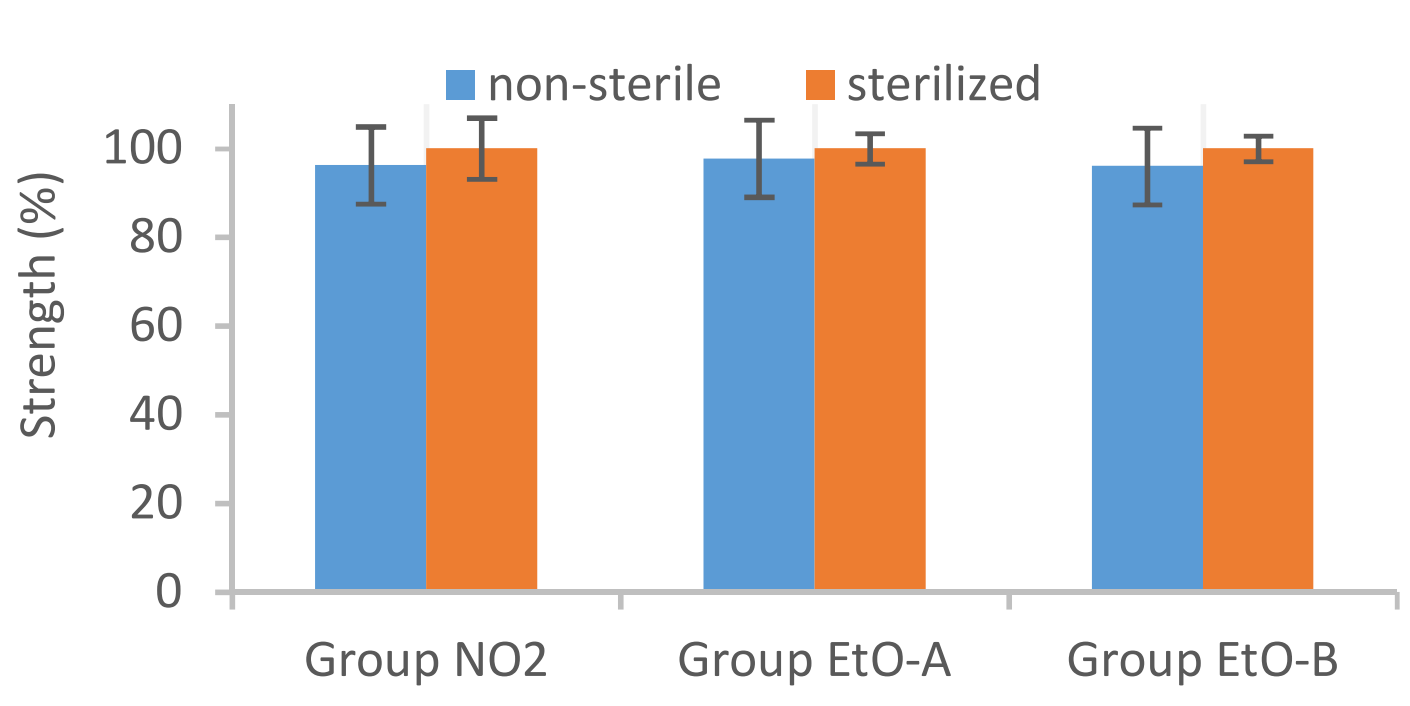
Effect on NO2 and EtO sterilization to Initial strength
Properties of Injection Molded Bioresorbable Glass Fiber Reinforced Composites
Huttunen, M., Ellä, V., Ahola, N., Heino, H., Lehtonen, T. Arctic Biomaterials Research and Development. ESB 2017 – The 28th Annual Conference of the European Society for Biomaterials.
Silica based bioresorbable glass fibers remarkably increase the strength properties of PLA based composites. In the past, both continuous and chopped fibers have been used as reinforcements. This study was focused in analysing injection molded PLA composites reinforced by chopped bioresorbable glass fibers. Investigational implant prototypes were successfully injection molded from 96L/4D PLA matrix and silica based bioresorbable glass fiber reinforcement. The adhesion between fibers and matrix was visible from SEM micrographs, which also revealed variable distribution of fiber reinforcement. In vitro degradation analysis revealed that in simulated conditions the studied composite and implant design combination retained pull-out force above 300N over 16 – 20 week follow-up.

Micro CT imaging showing X3 fiber distribution
Bioresorbable glass fiber reinforced composite as a material for prolonged bone fixations
Ellä, V., Ahola, N., Huttunen, M., Heino, H., Lehtonen, T. Arctic Biomaterials Research and Development. ESB 2017 – The 28th Annual Conference of the European Society for Biomaterials.
Bioresorbable glass fibre reinforced composites have previously been studied for bone fixation purposes. Glass fibre reinforced composite offers great advantage by increasing mechanical properties compared to polymer. Currently, there is a need to manufacture stronger bone fixation devices in order to treat fractures in load bearing indications of the human body. In this project, the objective was to produce very strong composites with altering degradation properties to be used either in sports medicine (arthroscopy) where shorter healing periods are required or in trauma and spine fixation where longer support times are needed.
As a conclusion, a very strong resorbable composite was developed that retains its strength for a desired period of time to be used for spinal and trauma devices that require a prolonged healing period. To our knowledge , for the first time the biodegradable material retained its strength this high at least 26 weeks. Thus, the property gap from 12 to 26 weeks has been resolved using this bioresorbable composite. The faster resorption time of the composite with PLGA 85/15 enables the use of this material in devices that are needed for operations with shorter support times such as sports medicine.

SEM images: Surface, crack surface and crack surface on top
Degradation characteristics of injection molded Evolvemer TCP30PLGA products
Arctic Biomaterials Research and Development
This white paper presented degradation properties for investigational suture anchor products composed of Evolvemer™ TCP30PLGA composite material. In vitro degradation analysis was conducted. Similar in vitro analyzes have been widely used to evaluate the degradation characteristics of similar composite materials and various implant prototypes. Composite materials composed of calcium phosphates (CaP) and PLA/PGA polymers have proven to be osteoconductive and they have been used with clinical success. One advantage of these materials over plain PLA/PGA polymers is that the CaP component (such as betaTCP) can provide a suitable base for cell bonding.
The outer dimension of the investigational suture anchor products composed of Evolvemer™ TCP30PLGA composite material gradually increased during the in vitro degradation, ending up 14% higher than the initial diameter at week 26. The mass loss started between weeks 26 and 52, being 35% of the initial mass at 104 weeks. The mechanical strength retention and the inherent viscosity data show that for the first 12 weeks of in vitro degradation, the mechanical properties remained above 60% of the initial dry strength and above 75% of the initial wet strength and the inherent viscosity was above 1.0 dl/g. This is still at the safe level compared to 0.8 dl/g threshold of more significant mechanical property reduction presented in reference studies.
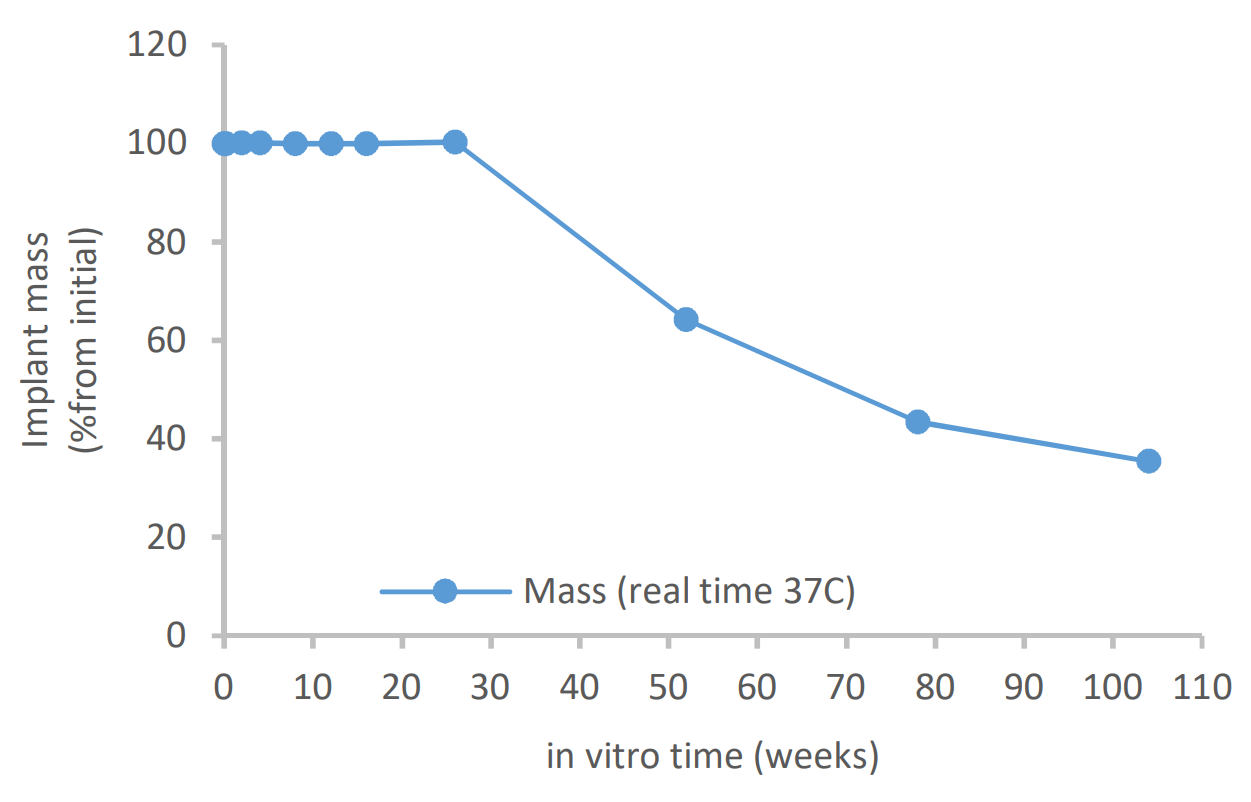
Mass loss during in vitro degradation of Evolvemer implant
Literature
Polylactide Composite Pins Reinforced with Bioresorbable Continuous Glass Fibers Demonstrating Bone-like Apatite Formation and Spiral Delamination Degradation
Cao, X.-Y., Tian, N., Dong, X., Cheng, C.-K. Polylactide Composite Pins Reinforced with Bioresorbable Continuous Glass Fibers Demonstrating Bone-like Apatite Formation and Spiral Delamination Degradation. Polymers 2019; 11; 812.
The emergence of polylactide composites reinforced with bioresorbable silicate glass fibers has allowed for the long-term success of biodegradable polymers in load-bearing orthopedic applications. However, few studies have reported on the degradation behavior and bioactivity of such biocomposites. The aim of this work was to investigate the degradation behavior and in vitro bioactivity of a novel biocomposite pin composed of bioresorbable continuous glass fibers and poly-L-D-lactide in simulated body fluid for 78 weeks. As the materials degraded, periodic spiral delamination formed microtubes and funnel-shaped structures in the biocomposite pins. It was speculated that the direction of degradation, from both ends towards the middle of the fibers and from the surface through to the bulk of the polymer matrix, could facilitate bone healing. Following immersion in simulated body fluid, a bone-like apatite layer formed on the biocomposite pins which had a similar composition and structure to natural bone. The sheet- and needle-like apatite nanostructure was doped with sodium, magnesium, and carbonate ions, which acted to lower the Ca/P atomic ratio to less than the stoichiometric apatite and presented a calcium-deficient apatite with low crystallinity. These findings demonstrated the bioactivity of the new biocomposite pins in vitro and their excellent potential for load-bearing applications.
In-vivo histocompatibility and osteogenic potential of biodegradable PLDLA composites containing silica-based bioactive glass fiber
Wu,P., Wang, Y., Sun, D. et al. Journal of Biomaterials Applications 2020;35(1); 59-71
The purpose of this two-year study was to evaluate the histocompatibility and osteogenic properties of a composite material consisting of poly(L-co-D,L lactide) (PLDLA) and silica-based bioactive glass fibers in vivo. PLDLA and PLDLA/silica-based bioactive glass fibers pins were implanted into the erector spinae muscles and femurs of beagles. Muscle and bone tissue samples were harvested 6, 12, 16, 26, 52, 78, and 104 weeks after implantation. Histology analysis was used to assess the histocompatibility, angiogenesis, and bone-implant contact. Micro-computed tomography was used to evaluate bone formation around the pins. Immunohistochemistry and western blotting revealed the expression level of the osteogenesis-related proteins. Addition of bioactive glass was demonstrated to possess better histocompatibility and reduce the inflammatory reactions in vivo. Moreover, PLDLA/silica-based bioactive glass fibers pins were demonstrated to promote angiogenesis and increase osteogenesis-related proteins expression, and thus played a positive role in osteogenesis and osseointegration after implantation. Our findings indicated that a composite of PLDLA and silicabased bioactive glass fiber is a promising biodegradable material for clinical use.
Overall, PLDLA/SGFs possessed better histocompatibility and reduced inflammatory reactions in two-year study in vivo, especially in the short term compared with PLDLA. The addition of bioactive glass fibers promoted angiogenesis and the expression of osteogenesis-related proteins, which increased osteogenesis and provided more stable anchorage after implantation. Therefore, PLDLA/SGFs composite is a promising biodegradable material for clinical use.
Mechanical and degradative properties of PLDLA biodegradable pins with bioactive glass fibers in a beagle model
Wang, Y., Wu, P., Sun, D., Luo, Y., Chen, C., Tang, Z., Liao, Y., Cao, X., Cheng, C. & Liu, W. BIomedical Materials 2020; 15(3)
The present study aimed to evaluate the mechanical and degradative properties of poly(L-co-D,L-lactic acid)/silicate bioactive glass fibers (PLDLA/SGFs) composite pins in vivo. Both PLDLA and PLDLA/SGFs pins were inserted into the erector spinae muscles and femurs of beagle dogs and were harvested 6, 12, 16, 26, 52, 78, and 104 weeks after insertion. Bone formation around the pins was evaluated by micro-computed tomography. Mechanical properties were measured by the shear strength test. Thermogravimetric analysis, differential scanning calorimetry, and gel permeation chromatography were used to assess the degradation of these materials. The surface and cross-sectional morphology of both pins were observed using a scanning electron microscope. The experimental data demonstrated that PLDLA/SGFs pins can support new bone formation due to the influence of bioactive glass fibers. PLDLA/SGFs composite pins had higher initial shear strength and were relatively stable for at least 26 weeks. The addition of bioactive glass fibers accelerated the degradation rate of the composite pins. Thus, PLDLA/SGFs composite pins have promising potential for bone fixation applications.
Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering
Gerhardt, L-C., Boccaccini, A.R. Materials 2010; 3(7); 3867-3910.
Traditionally, bioactive glasses have been used to fill and restore bone defects. More recently, this category of biomaterials has become an emerging research field for bone tissue engineering applications. Here, we review and discuss current knowledge on porous bone tissue engineering scaffolds on the basis of melt-derived bioactive silicate glass compositions and relevant composite structures. Starting with an excerpt on the history of bioactive glasses, as well as on fundamental requirements for bone tissue engineering scaffolds, a detailed overview on recent developments of bioactive glass and glass-ceramic scaffolds will be given, including a summary of common fabrication methods and a discussion on the microstructural-mechanical properties of scaffolds in relation to human bone (structure-property and structure-function relationship). In addition, ion release effects of bioactive glasses concerning osteogenic and angiogenic responses are addressed.
Bioactive glass fiber/polymeric composites bond to bone tissue
Marcolongo, M., Ducheyne, P., Garino, J., Schepers, E. Journal of Biomedical Materials Research 1998; 39(1); 161-170.
Bioactive glass fibers were investigated for use as a fixation vehicle between a low modulus, polymeric composite and bone tissue. In an initial pilot study, bioactive glass fiber/polysulfone composites and all-polysulfone control rods were implanted into the rabbit tibia; the study was subsequently expanded with implantation into the rabbit femur. Bone tissue exhibited direct contact with the glass fibers and adjacent polymer matrix and displayed a mechanical bond between the composite and bone tissue after six weeks implantation. Interfacial bond strengths after six weeks implantation averaged 12.4 MPa, significantly higher than those of the all-polymer controls. Failure sites for the composite at six weeks generally occurred in the bone tissue or composite, whereas the failure site for the polymer implants occurred exclusively at the implant/tissue interface. The bioactive glass fiber/polysulfone composite achieved fixation to bone tissue through a triple mechanism: a bond to the bioactive glass fiber, mechanical interlocking between the tissue and glass fibers, and close apposition and possible chemical bond between the portions of the polymer and bone tissue. This last mechanism resulted from an overspill of bioactivity reactions from the fibers onto the surface of the surrounding polymer which we call the “halo” effect.
A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics
Hoppe, A., Güldal, N.S., Boccaccini, A.R. Biomaterials 2011; 32; 2757-2774
Several inorganic materials such as special compositions of silicate glasses, glass-ceramics and calcium phosphates have been shown to be bioactive and resorbable and to exhibit appropriate mechanical properties which make them suitable for bone tissue engineering applications. However, the exact mechanism of interaction between the ionic dissolution products of such inorganic materials and human cells are not fully understood, which has prompted considerable research work in the biomaterials community during the last decade. This review comprehensively covers literature reports which have investigated specifically the effect of dissolution products of silicate bioactive glasses and glass-ceramics in relation to osteogenesis and angiogenesis. Particularly, recent advances made in fabricating dense biomaterials and scaffolds doped with trace elements (e.g. Zn, Sr, Mg, and Cu) and investigations on the effect of these elements on the scaffold biological performance are summarized and discussed in detail.
Clearly, the biological response to artificial materials depends on many parameters such as chemical composition, topography, porosity and grain size. This review, however, focuses only on the ion release kinetics of the materials and the specific effect of the released ionic dissolution products on human cell behaviour, providing also a scope for future investigations and identifying specific research needs to advance the field. The biological performance of pure and doped silicate glasses, phosphate based glasses with novel specific compositions as well as several other silicate based compounds are discussed in detail. Cells investigated in the reviewed articles include human osteoblastic and osteoclastic cells as well as endothelial cells and stem cells.
A comprehensive analysis of the available literature on the effect of ionic dissolution products of inorganic bioactive glasses and glass-ceramics (silicate and phosphate systems) in the context of bone tissue engineering has been presented. Taking into account the experimental evidence summarized in this review and the discussion above, a significant challenge for developing scaffolds for bone regeneration based on these inorganic materials is to achieve controlled ion dissolution and release..
Bioactive glass in tissue engineering
Rahaman, M.N., Day, D.E., Bal, B.S., Fu, Q., Jung, S.B., Bonewald, L.F., Tomsia, A.P. Acta Biomaterialia 2011; 7(6); 2355-2373.
This review focuses on recent advances in the development and use of bioactive glass for tissue engineering applications. Despite its inherent brittleness, bioactive glass has several appealing characteristics as a scaffold material for bone tissue engineering. New bioactive glasses based on borate and borosilicate compositions have shown the ability to enhance new bone formation when compared to silicate bioactive glass. Borate-based bioactive glasses also have controllable degradation rates, so the degradation of the bioactive glass implant can be more closely matched to the rate of new bone formation. Bioactive glasses can be doped with trace quantities of elements such as Cu, Zn and Sr, which are known to be beneficial for healthy bone growth. In addition to the new bioactive glasses, recent advances in biomaterials processing have resulted in the creation of scaffold architectures with a range of mechanical properties suitable for the substitution of loaded as well as non-loaded bone.
While bioactive glass has been extensively investigated for bone repair, there has been relatively little research on the application of bioactive glass to the repair of soft tissues. However, recent work has shown the ability of bioactive glass to promote angiogenesis, which is critical to numerous applications in tissue regeneration, such as neovascularization for bone regeneration and the healing of soft tissue wounds. Bioactive glass has also been shown to enhance neocartilage formation during in vitro culture of chondrocyte-seeded hydrogels, and to serve as a subchondral substrate for tissue-engineered osteochondral constructs. Methods used to manipulate the structure and performance of bioactive glass in these tissue engineering applications are analyzed.
Long-term study on the osteogenetic capability and mechanical behavior of a new resorbable biocomposite anchor in a canine model
Cao., X.Y., Chen, C., Tian, N., Dong, X., Liang, X., Xu L-J. & Cheng, C-K. Journal of Orthopaedic Translation 2020; 21; 81-90
Biodegradable suture anchors are commonly used for repairing torn rotator cuffs, but these biodegradable materials still suffer from low mechanical strength, poor osteointegration, and the generation of acidic degradation byproducts. The purpose of this study was to evaluate the long-term mechanical behavior and osteogenetic capabilities of a biocomposite anchor injection molded with 30% β-tricalcium phosphate microparticles blended with 70% poly (L-lactide-co-glycolide) (85/15). This study investigated in vitro degradation and in vivo bone formation in a canine model. The initial mechanical behavior, mechanical strength retention with degradation time, and degradation features were investigated.
The results showed that the biocomposite anchor had sufficient initial mechanical stability confirmed by comparing the initial shear load on the anchor with the minimum shear load borne by an ankle fracture fixation screw, which is considered a worst-case implantation site for mechanical loading. The maximum shear load retention of the biocomposite anchor was 83% at 12 weeks, which is desirable, as it aligns with the rate of bone healing. The β-tricalcium phosphate fillers were evenly dispersed in the polymeric matrix and acted to slow the degradation rate and improve the mechanical strength of the anchor. The interface characteristics between the β-tricalcium phosphate particles and the polymeric matrix changed the degradation behavior of the biocomposite. Phosphate buffer saline was shown to diffuse through the interface into the biocomposite to inhibit the core accelerated degradation rate. In vivo, the addition of β-tricalcium phosphate induced new bone formation. The biocomposite material developed in this study demonstrated improved osteogenesis in comparison to a plain poly (L-lactide-co-glycolide) material. Neither anchor produced adverse tissue reactions, indicating that the biocomposite had favorable biocompatibility following long-term implantation. In summary, the new biocomposite anchor presented in this study had favorable osteogenetic capability, mechanical property, and controlled degradation rate for bone fixation.
Quantitative comparison of bone growth behavior in granules of Bioglass®, A-W glass-ceramic, and hydroxyapatite
Oonishi, H., Hench, L.L., Wilson, J., Sugihara, F., Tsuji, E., Matsuura, M., Kin, S., Yamamoto, T. & Mizokawa, S. Journal of Biomedical Materials Research 2000; 51(1), 37-46
The hypothesis that bioactive glass particulate increases the rate of bone proliferation over that of synthetic hydroxyapatite and bioactive glass-ceramic was tested in these experiments. Three types of bioactive particles–45S5 Bioglass®, synthetic hydroxyapatite, and A-W glass-ceramic—were implanted in 6-mm-diameter holes drilled in the femoral condyles of mature rabbits. Bone growth rate was measured using an image processor. 45S5 Bioglass® produced bone more rapidly than either A-W glass-ceramic or hydroxyapatite. At the later time periods, 45S5 Bioglass® was resorbed more quickly than A-W glass-ceramic. Synthetic hydroxyapatite was not resorbed at all. Backscattered electron imaging suggested that the resorption process occurred by solution-mediated dissolution, which produced chemical changes in the enclosed particulate. It was concluded that the rate of bone growth correlates with the rate of dissolution of silica as the particles resorb.




